Prof. Tongiorgi’s laboratory has given a major contribution to the understanding of the biological functions of Brain-Derived Neurotrophic Factor (BDNF). They demonstrated that BDNF splice variants provide a spatial and quantitative code for local expression and selective morphological shaping of dendrites during development. They investigated BDNF proteolytic forms as biomarkers of chronic stress, depression, multiple sclerosis, autism and cognitive impairment in schizophrenia. The laboratory is part of the “Italian network on BDNF” (In-BDNF). Current research is focused on the rescue of neuronal atrophy by exploiting the BDNF variants expression code and pharmacological treatments and its monitoring by serum/CSF biomarkers in neurodevelopmental disorders, in particular the Rett syndrome.
1) Pharmacological rescue of neuronal atrophy in Rett Syndrome
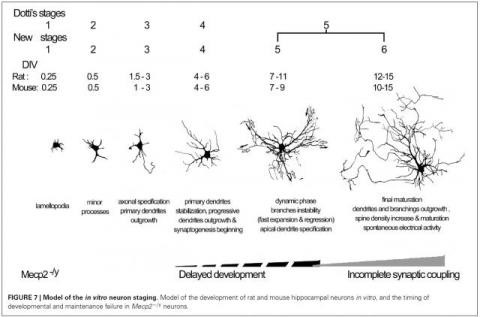
Brain weight loss, shrinkage of the cortex with reduced soma and dendritic arborization in absence of neurodegenerative processes is a typical feature of the neurodevelopmental disorder Rett syndrome, an X-linked genetic disease mainly caused by mutations in the MeCP2 (Methyl-CpG binding protein-2) gene. Current major hypotheses for the causes of neuronal atrophy involve intrinsic (cell-autonomous) and extrinsic (non-cell autonomous) factors including impaired neurotrophic support due to reduced BDNF, decreased monoamine neurotransmission, increased oxidative stress and impaired neuron-glia crosstalk. Accordingly, we are testing the antidepressant Mirtazapine and other drugs to enhance monoamines and neurotrophic factors, reduce oxidative stress and rescue neuronal atrophy. The project is taking advantage of in vivo and in vitro models using a MeCP2 knock-out mouse and iPSCs-derived neuronal cultures produced from patient fibroblasts.
Translational Outcomes:
1) The lab is home of the DRUG SCREENING CENTER FOR THE RETT SYNDROME, based on highly standardized mouse or rat cell-based model of neural development in vitro suitable for screening of chemical or natural compounds by high- content imaging analysis (Nerli et al. Scientific Reports, 2020 -https://www.nature.com/articles/s41598-020-59268-w ).
2) Test of drugs and natural compounds in MeCP2 knock-out mouse in vivo and in vivo models to speed up translation towards clinical trials (repositioning approach – Bittolo et al., 2016 - http://dx.doi.org/10.1038/srep19796; Flores-Gutierrez et al., 2020 https://doi.org/10.1186/s11689-020-09328-z).
2) Neurotrophic factors and cytokines as biomarkers of neuronal atrophy and its recovery
Research activities are focused on the analysis of neurotrophic factors (particularly BDNF) and cytokines in serum and CSF to monitor pharmacological treatments and neuro-rehabilitative therapies in patients with neuronal atrophy associated with injury, neuropsychiatric or neurodegenerative diseases.
Translational Outcomes:
1) Development and validation of innovative in vitro assays for clinical biomarkers of neurological and neuropsychiatric disorders and monitoring of pharmacological treatments or rehabilitative therapies.
2) The laboratory has collaborated with various private companies as beta-tester of pre-commercial kits.
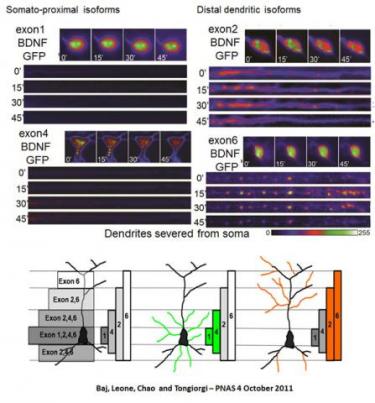
3) The “spatial and quantitative code model” of BDNF splice variants
The neurotrophin Brain-Derived Neurotrophic Factor (BDNF) is a key morphoregulatory molecule in neuronal development and plasticity. Transcription of the bdnf gene leads to 34 transcripts in humans and 22 in rodents, each with the same coding region (CDS), one out of 11 different 5’UTRs, and either a short or a long 3’UTR. To explain the biological role of these multiple BDNF splice variants we proposed the “spatial and quantitative code” model. This model is based on our finding that BDNF mRNA variants become spatially segregated in response to neuronal activation within three distinct subcellular domains: the soma (exons 1, 3, 5, 7, 8, 9a), the proximal (exon 4) or the distal (exon 2, 6) dendrites. We also showed that each mRNA variant has a different translatability and produces different quantity of BDNF in response to different neurotransmitters. Current research is focused on understanding the mechanisms of BDNF mRNA variants segregation and local translation and exploitation of the BDNF code to rescue dendritic atrophy in various neurological diseases.
Translational Outcomes:
1) Patented high throughput cell-based assay to screen for natural and synthetic compounds able to modulate BDNF protein translation (PCT/EP2010/067081).
2) BDNF spatial code database in human and rodent brain for rational design of strategies to rescue neuronal atrophy and cognitive impairment in different brain areas.