The team is involved in the following main lines of research:
1. Risk characterization of algal biotoxins and setting up new methods for their detection in seafood, the producing organisms and/or seawater, which include: (a) in vivo/in vitro studies on the toxic effects of algal toxins and their mechanisms of action; (b) set up of new methods for the detection and quantification of algal toxins (structural assays for their detection in seafood, microalgae and seawater; functional assays, based on their mechanism(s) of action), as well as of biosensors to improve their sensitivity.
2. Pharmacological and toxicological investigation of herbal drugs for their use in health care and as source of new drugs, which include: (a) in vitro and in vivo studies on medicinal plant products to assess their efficacy and toxicity and to identify the active principles using a bioassay-oriented fractionation approach); (b) in vitro studies on the mechanisms of action of the active or toxic principles.
3. Toxicological investigation on graphene and graphene based nanomaterials. Grphene and grapheme based materials are investigated for their irritant and/or sensitizing potential of after cutaneous exposure using in vitro studies on human HaCaT skin keratinocytes and reconstituted epidermis, as well as in vivo models. Moreover, these nanomaterials are studied from an ecotoxicological point of view, investigating their effects on the marine crustacean Artemia franciscana, a significant link in the food chain and a well-known organism model for ecotoxicological assessment of nanoparticles.
1) Set up of rapid and sensitive screening methods to detect algal toxins
Marine algal toxins represent a worldwide sanitary and economic problem, being responsible for human poisonings after consumption of seafood contaminated by these compounds or adverse effects after inhalational exposure to marine aerosol and/or direct contact with seawater. In the last decades, the highly toxic palytoxins (PLTXs; mainly the analogue ovatoxin-a) were frequently identified in the Mediterranean Sea during Ostreopsis cf. ovata blooms.
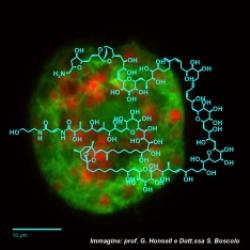 Thus, the project is aimed to set up new methods for an easy, rapid and sensitive detection of palytoxins in seafood and directly in microalgae. A new immunoenzymatic assay (cell-based ELISA) has been developed for PLTX quantitation in mussels.
Thus, the project is aimed to set up new methods for an easy, rapid and sensitive detection of palytoxins in seafood and directly in microalgae. A new immunoenzymatic assay (cell-based ELISA) has been developed for PLTX quantitation in mussels.
This assay will allow an easy and rapid screening of seafood for the presence of these biotoxins, useful for self-monitoring by producers as well as for institutional monitoring programs before confirmatory chemical analyses in case of positive results. The assay will be useful also in monitoring programs to identify PLTXs-producing microalgae prior to blooming and forecast shellfish contamination in the appearance Ostreopsis: in fact, similarly to other dinoflagellates, Ostreopsis cells do not always produce the same amounts of toxins, if any. Thus, the detection of PLTXs in microalgae could give an early warning, reducing sanitary problems for the consumers as well as unnecessary economic losses for the producers.
Similarly, it will avoid adverse effects in humans during the recreational or occupational activities in coastal areas concomitantly to Ostreopsis blooms and the negative economic impact on tourism industry, such as that in Provence (France) after hotel cancellations at the first report on Ostreopsis cells in seawater.
Part of the research is carried out also within the project “EMERTOX”, supported by a Marie Skodowska-Curie Research and Innovation Staff Exchanges (RISE) action within the H2020 work programme, aimed to map the actual situation in emergent marine toxins and the producing organisms, develop new approaches to assess their occurrence and predict the possible future scenarios in the framework of global warming.
2) Toxicity studies on algal toxins
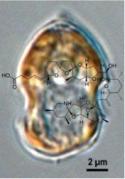
The in vitro and in vivo toxicity studies on algal toxins are partly focused on ovatoxin-a, the main analog of palytoxin detected in the Mediterranean area, with the aim of identifying and characterizing the hazard, necessary for the risk assessment of this compound as seafood contaminant.
Moreover, in collaboration with the Food and Drug Administration (FDA, USA), toxicity studies are carried out on azaspiracido59 (AZA59), a polyether of algal origin that can accumulate in edible mussels and cause a serious gastrointestinal syndrome in humans.
In addition, the in vivo and in vitro toxic effects of dihydro-dinophysistoxin-1, a new structural analogue of diarrheal toxins belonging to the okadaic acid group, are going to be studied within a project granted by US National Ocean Service (NOS)-National Oceanic and Atmospheric Administration (NOAA)-National Centers for Coastal Ocean Science (NCCOS) - ECOHAB program.
3) Anti-inflammatory principles from plant kingdom
Plant kingdom is a valuable source of a wide variety of bioactive compounds even though only a limited number of plant-derived products are used as therapeutics or as leads for new drugs discovery. On the other hand, several species are used by the traditional medicine of different geographical areas but experimental studies are often needed to confirm their pharmacological properties and to identify the relevant active principles. The project is aimed to identify and exploit the use of medicinal and food plants as source of anti-inflammatory compounds potentially useful in the treatment of inflammatory based diseases.
 In particular, the anti-inflammatory activity of volatile, semi-volatile and non-volatile components of plants selected on the basis of ethnobotanical and phytochemical parameters is investigated following a bioassay-oriented fractionation approach, aimed to identify the active principles. The latter are studied in detail to identify their mechanisms of action and verify possible toxic effects, in view of a therapeutic use.
In particular, the anti-inflammatory activity of volatile, semi-volatile and non-volatile components of plants selected on the basis of ethnobotanical and phytochemical parameters is investigated following a bioassay-oriented fractionation approach, aimed to identify the active principles. The latter are studied in detail to identify their mechanisms of action and verify possible toxic effects, in view of a therapeutic use.
4) Toxicity of graphene family nanomaterials
In the last years, graphene-based nanomaterials (GBNs) have attracted both academic and industrial interest due to their extraordinary physicochemical properties, which make them promising platforms for several applications in the field of nanotechnology and biomedicine. However, despite the huge GBNs technologies progress, little is known about their impact on human health, so far. In particular, the cutaneous toxicity of GBNs remains largely unexplored despite skin contact can be considered one of the major exposure routes to GBNs during their production and use. Thus, within the EU Horizon 2020 project “GRAPHENE FLAGSHIP”, WP4 “Health and Environment”, the toxic effects of graphene and related nanomaterials after cutaneous exposure are investigated through in vitro studies using human skin HaCaT keratinocytes and reconstituted epidermis, as well as in vivo models to evaluate their irritant and/or sensitizing properties. The use of cell based in vitro systems for safety evaluations are used also to shift the expected results towards the mechanistic understanding of the toxic effects.
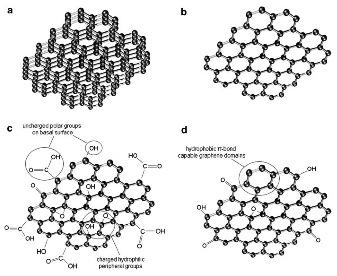
Members of the Graphene Family Nanomaterials: (a) few-layer-graphene; (b) graphene nanosheet; (c) graphene oxide; (d) reduced graphene (Jastrzebska et al. - J Nanopart Res 14: 1320-41, 2012).
5) Effects of graphene-based nanomaterials on Artemia franciscana after long-term exposure
Graphene based nanomaterials (GBNs) and algal toxins are studies also from an ecotoxicological point of view for their effects on the marine crustacean Artemia franciscana, a significant link in the food chain and a well-known organism model for ecotoxicological studies. In particular, short and long-term toxicity studies are aimed to evaluate different endpoints in A. franciscana exposed to GBNs or algal toxins, to shed light on their ecotoxicological impact.