The research programs coordinated by Dr. Violetta Borelli are focused on the study of the role of inflammation/inflammatory cells in the pathogenetic activity of asbestos fibres and in other human diseases. These activities are organized in 3 main lines of research:
1) Identify an association between certain genetic polymorphisms (inflammasome related) and individual ability to form asbestos bodies after asbestos exposure.
2) Study of the mechanisms of interaction of asbestos fibres with cellular compartments of inflammatory cells (mast cells).
3) Mast cells in human health and disease: the role of mast cells and their mediators in endometriosis and in periodontal disease.
Current Projects
1) Inflammation and lung iron homeostasis and pleural mesothelioma
The presence of asbestos bodies in lung parenchyma is considered a histopathologic hallmark of past exposure to asbestos fibers of which there was a population of longer fibers. The mechanisms underlying asbestos body formation are complex, involving inflammatory responses and iron (Fe) metabolism. Thus the responsiveness to asbestos body formation is variable with some individuals appearing to be poor asbestos body formers. The aim of this project is to disclose the possible role of genetic variants of genes encoding inflammasome and iron metabolism proteins in the ability to form asbestos bodies in the population from North East Italy, who died after having developed malignant pleural mesothelioma, lung cancer or asbestosis. Our preliminary findings suggest that the NLRP1 inflammasome might contribute in the development of lung asbestos bodies in mesothelioma affected subjects. It is postulated that the NLRP1 missense variant may be considered as one of the possible host genetic factors contributing to individual variability in coating efficiency, which needs to be taken when assessing occupational exposure to asbestos.
2) Study of the mechanisms of interaction of mineral fibers with cellular compartments.
Exposure to mineral fibers is of great relevance for human health. A key event in this process is the interaction with inflammatory cells and the following generation of pro-inflammatory factors. Mast cells (MC) have been shown to interact with titanium oxide (TiO2)and asbestos fibers. In this project we analyze the response of rat peritoneal MC challenged with the asbestos crocidolite and nanowires of TiO2 compared with that induced by wollastonite employed as control fiber. The amount of secreted enzymes is evaluated together with the activity of fiber associated enzymes. The ultrastructural morphology of fiber-interacting RPMC is analyzed with electron microscopy.
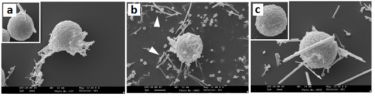
Scanning electron microscope (SEM) appearance of RPMC exposed to mineral fibers: a9 asbestos crocidolite, b) wollastonite and c) TiO2. Magnification: bars b = 3 µm; bars a and c = 1µm.
3) The role of mast cells and their mediators in endometriosis and human diseases
Mast cells are primarily known for their role in defense against pathogens, particularly bacteria; neutralization of venom toxins; and for triggering allergic responses and anaphylaxis. In addition to these direct effector functions, activated mast cells rapidly recruit other innate and adaptive immune cells and can participate in "tuning" the immune response. In this two projects we focus on the roles of mast cells in human two human diseases.
- In human periodontal disease there is an increase in the number of mast cells that may be participating either in the destructive events or in the defense mechanism of periodontal disease via secretion of cytokines and proteolytic enzymes. The aim of this project is to quantify the main mast cells protease, tryptase, in the peri-implant sulcus fluid of healthy and affected sites, whether it correlate degree of inflammation and clinical features of periodontium (in collaboration with the Department of Surgical, Medical and Health Sciences, University of Trieste, Trieste, Italy).
- Increasing evidence supports an involvement of mast cells either in the inflammatory process of endometriosis or in infertility, with MC mediators directly suppressing sperm motility in a potentially reversible manner. In this project we are evaluating the MCs/basophils population and their mediators in the peritoneal environment/fluid of infertile patients with endometriosis and their impact on human sperm motility. Furthermore an in vitro model of mast cells-sperm interaction in the peritoneal environment has been set up and evaluated from a functional and a morphological point of view (in collaboration with Institute for Maternal and Child Health, IRCCS "Burlo Garofolo", 34137 Trieste, Italy).
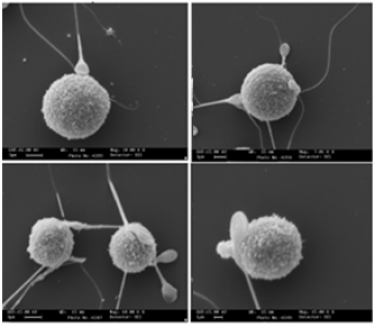
Low-magnification SEM examination of adherence of human sperm by the human MC line LAD2